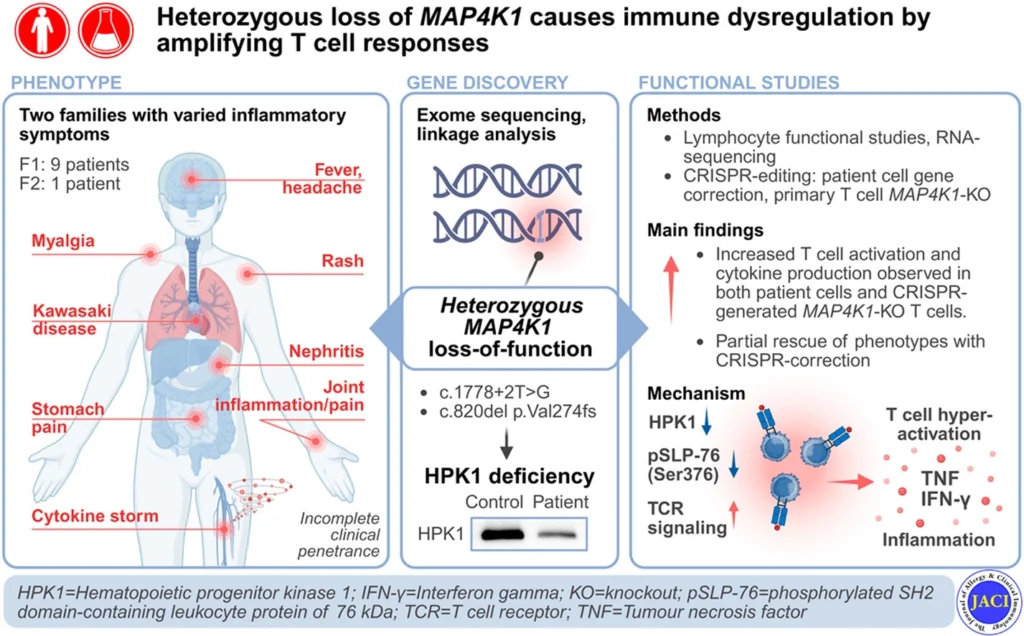
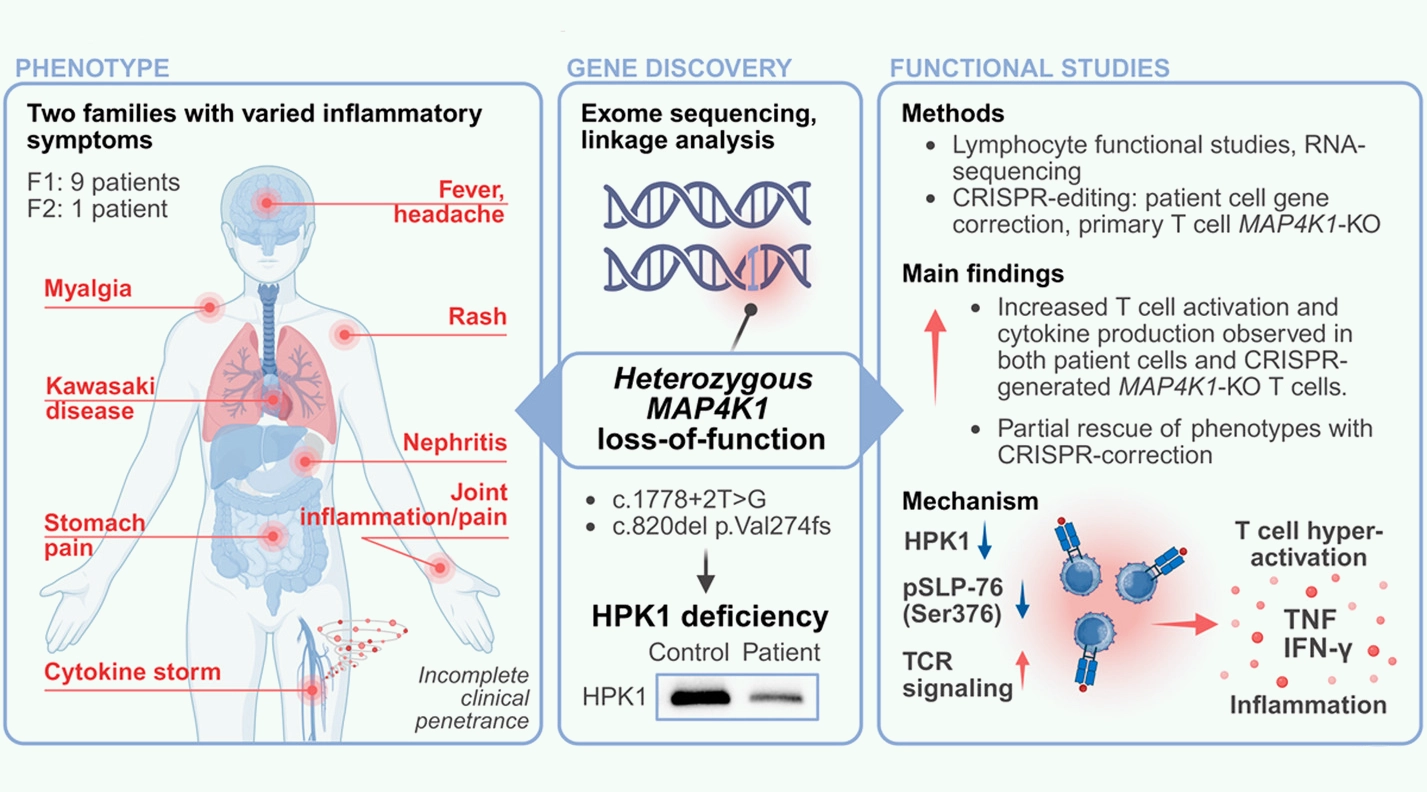
We’re excited to share a new paper published in the Journal of Allergy and Clinical Immunology: Heterozygous loss of MAP4K1 causes immune dysregulation by amplifying T cell responses.
The study was initiated and led by the Saarela group, with our team joining along the way and contributing throughout the project. It was a great collaboration, and the corrected proof is now available on ScienceDirect.
Abstract
Background:
MAP4K1 encodes Hematopoietic Progenitor Kinase 1 (HPK1), a serine/threonine kinase that negatively regulates T cell receptor (TCR) signaling via phosphorylation of the adaptor proteins SLP-76 and Gads. While common MAP4K1 variants have been implicated in polygenic immune-mediated diseases, the impact of rare germline variants on human immunity remains undefined.
Objective:
To investigate the immunological and functional consequences of HPK1 deficiency in individuals with suspected Inborn Errors of Immunity (IEI).
Methods:
We performed genomic linkage analysis and exome sequencing to identify disease-associated variants in IEI patients. Immunophenotyping, RNA-sequencing and functional assays were conducted on patient-derived lymphocytes, complemented by CRISPR-Cas9-mediated MAP4K1 disruption and correction in primary T cells.
Results:
Heterozygous MAP4K1 loss-of-function variants were identified in two kindreds presenting with diverse immune dysregulatory symptoms, including recurrent fevers, inflammatory arthritis, EBV-related complications, and nephritis. These variants led to reduced HPK1 protein levels and SLP-76Ser376 phosphorylation. While lymphocyte development was largely preserved, T cells from HPK1-deficient individuals displayed hyperresponsiveness to TCR stimulation, characterized by elevated secretion of proinflammatory cytokines, particularly interferon-γ (IFN-γ) and tumor necrosis factor (TNF). CRISPR-mediated knockout recapitulated, and variant-correction partially reversed this phenotype.
Transcriptomic profiling of stimulated CD4+ T cells further revealed upregulation of immune signaling pathways—including NF-κB, JAK/STAT, and AP-1—as well as increased expression of multiple T cell cytokines, consistent with enhanced TCR signaling and T cell responses in HPK1-deficient individuals.
Conclusion:
HPK1 deficiency, caused by heterozygous loss of MAP4K1, is a novel monogenic cause of immune dysregulation. Increased T cell activation and pro-inflammatory cytokine production are implicated in disease pathogenesis.